
darolutamide in ENZ-R CRPC provide a rationale for further
evaluation of darolutamide in ENZ-R CRPC in the clinic.
Second, a variety of AR mutants are induced under selective
pressures of AR pathway inhibition in CRPC patients and
darolutamide remains an antagonist even in those confer-
ring agonism to ENZ. In accordancewith a precision urologic
oncology approach, treatment predictors such as ctDNA-
defined AR mutational status will help define specific and
actionable AR mutants or variants to help enrich patient
selection and guide therapy. Based on our results, darolu-
tamide might have its place in therapeutic considerations in
this precision urologic oncology setting.
Author contributions:
Martin E. Gleave had full access to all the data in
the study and takes responsibility for the integrity of the data and the
accuracy of the data analysis.
Study concept and design:
Borgmann, Lejeune, Cherkasov, Gleave.
Acquisition of data:
Borgmann, Lallous, Ozistanbullu, Beraldi, Paul, Dalal,
Fazli.
Analysis and interpretation of data:
Borgmann, Lallous, Ozistanbullu,
Beraldi, Paul, Dalal, Fazli, Haferkamp, Cherkasov, Gleave.
Drafting of the manuscript:
Borgmann, Lallous, Ozistanbullu, Cherkasov,
Gleave.
Critical revision of the manuscript for important intellectual content:
Beraldi, Paul, Dalal, Fazli, Haferkamp, Lejeune.
Statistical analysis:
Borgmann, Lallous, Ozistanbullu, Paul, Fazli.
Obtaining funding:
Cherkasov, Gleave.
Administrative, technical, or material support:
None.
Supervision:
Haferkamp, Cherkasov, Gleave.
Other:
None.
Financial disclosures:
Martin E. Gleave certi
fi
es that all con
fl
icts of
interest, including speci
fi
c
fi
nancial interests and relationships and
af
fi
liations relevant to the subject matter or materials discussed in the
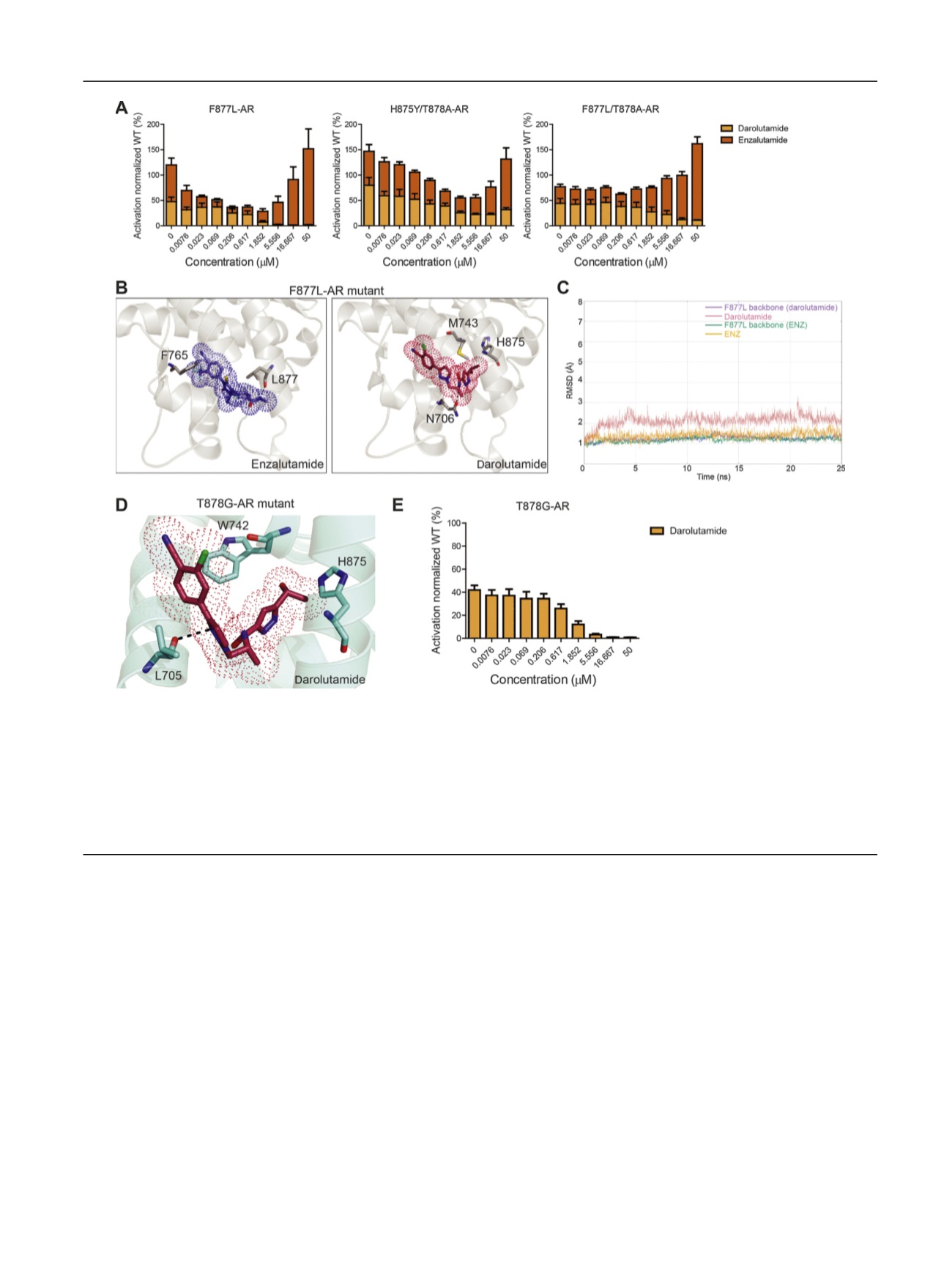
[(Fig._2)TD$FIG]
Fig. 2
–
Effect of enzalutamide (ENZ) and darolutamide (ODM-201) on castration-resistant prostate cancer-associated androgen receptor (AR) mutations
and cheminformatics in silico modeling of drug
’
s mode of action. AR transactivation as assessed using luciferase assay 24 h after treatment with
darolutamide or ENZ in AR-mutants F877L, F877L/T878A, and H875Y/T878A at indicated doses (A). PC3 cells were transfected with the mutated AR
construct and a reporter plasmid pARR3-tk-luciferase. Pooled means of triplicate experiments are plotted plus or minus the standard error of the
mean. (B) Binding pose of ENZ (left) versus darolutamide (right) within the F877L mutant
’
s androgen-binding-site, reveals how altered conformations
within the pocket lead to distinct protein
–
ligand interactions. (C) Root mean squared deviation (RMSD) analysis comparing the binding conformations
of ENZ and darolutamide within the androgen-binding-site pocket of F877L mutant. (D) Binding pose of darolutamide in the androgen binding site of
the previously uncharacterized AR mutant T878G. (E) Darolutamide inhibited the transcriptional activity of T878G mutant previously described to
induce agonism to ENZ, bicalutamide, and hydroxyflutamide.
WT = wild type.
E U R O P E A N U R O L O GY 7 3 ( 2 0 18 ) 4
–
8
7
















