
1.
Introduction
A key clinical outcome of nephron-sparing surgery (NSS) is
the detrimental effect of surgery on renal function,
particularly when temporary renal vascular clamping is
applied to optimize visualization for excision, repair, and
hemostasis
[1] .Modifiable factors employed to minimize
detrimental effects include the utilization of cold ischemia,
limiting the duration of renal artery occlusion, and
intraoperative use of renal protective agents prior to renal
ischemia.
Mannitol is an osmotic diuretic used during NSS to
mitigate the effects of ischemic renal injury. Cited evidence
has been drawn from clinical experience and preclinical
animal studies
[1]. Associated prostaglandin-mediated
vasodilatory effects, the release of atrial natriuretic peptide,
or a combination of both mechanisms have been suggested
as factors that increase renal blood flow
[2,3] .Others
include free-radical scavenging, reduction of renin produc-
tion, and expansion of intravascular volume
[4].
To our knowledge, no randomized trial exists that
supports the use of mannitol during NSS. A recent
randomized trial presented at the American Urological
Association 2017 meeting confirms our findings in mini-
mally invasive NSS
[5] .On the contrary, renal physiology
studies indicate that a competitive mechanism from
increased metabolic demand might actually have a detri-
mental effect on renal function
[6] .Comparative retrospective data has raised further
questions about the clinical value of mannitol during NSS.
Functional outcomes measured by estimated glomerular
filtration rate (eGFR) within 6 mo after surgery were no
different between 164 surgical patients who received
mannitol versus 121 who did not
[7] .Based on this data,
we conducted a randomized controlled trial to compare the
effects of mannitol versus placebo on postoperative kidney
function in patients undergoing NSS for renal cell cancer.
2.
Materials and methods
2.1.
Trial design and participants
The trial was a prospective, randomized, placebo-controlled, double-
blind, clinically integrated trial. Patients were recruited between July
2012 and July 2015. Approximately 46% of eligible patients consented
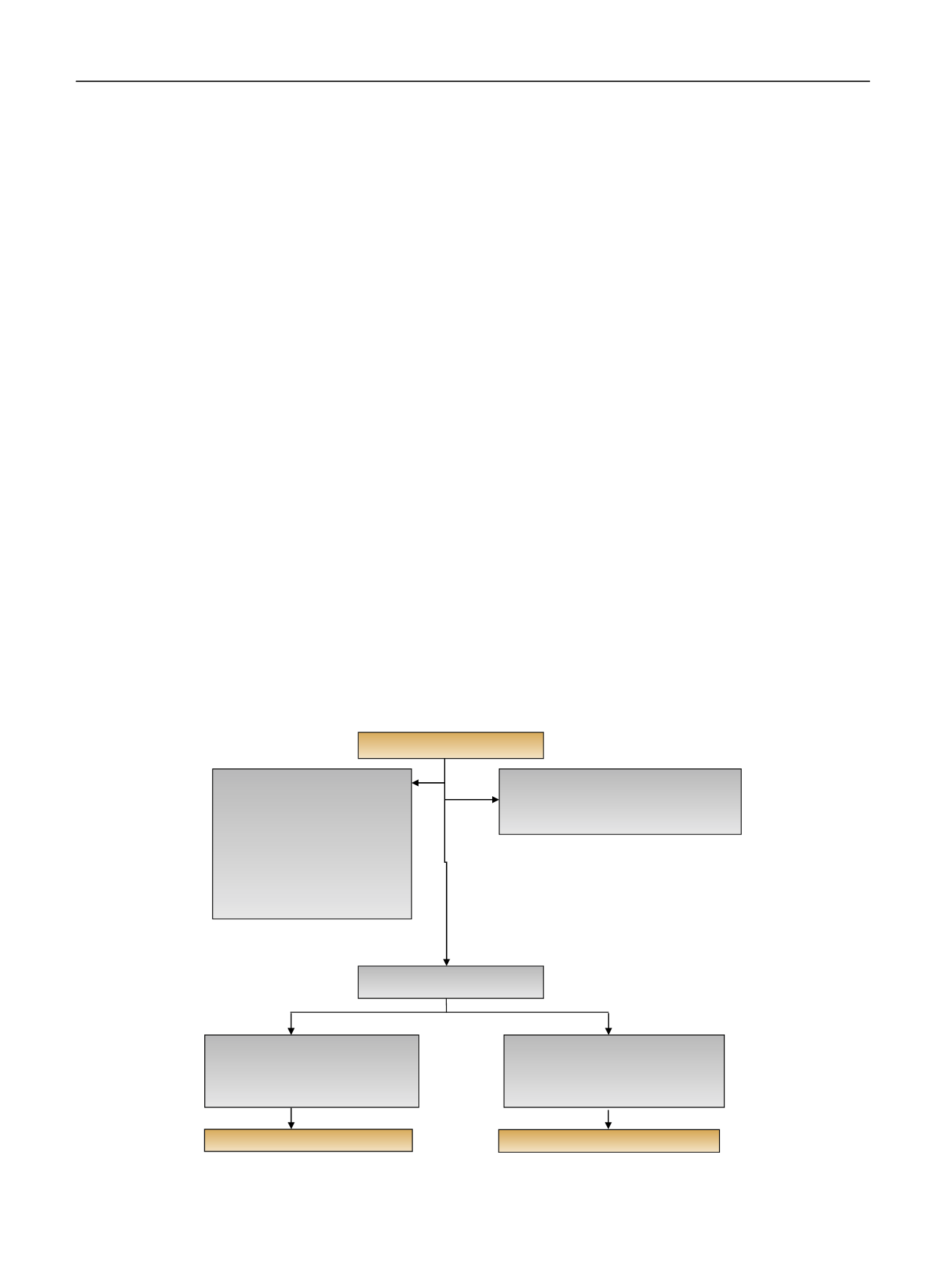
( Fig. 1).
Eligible patients had a renal mass, were 18 yr old, medically cleared
for NSS during which renal ischemia was anticipated, and had a pre-
operative eGFR 45 ml/min/1.73 m
2
(Chronic Kidney Disease Epidemiol-
ogy Collaboration). NSS was performed using an open or minimally
invasive approach. Exclusion criteria included allergy to mannitol, severe
renal function impairment (stage 3B) de
fi
ned as eGFR 45 ml/min/
1.73 m
2
, or multiple surgical procedures combined with NSS.
Randomization was strati
fi
ed by preoperative eGFR (
<
60 vs 60 ml/
min/1.73 m
2
) and surgical approach (open vs minimally invasive), and
patients were assigned in a 1:1 ratio to receivemannitol or normal saline.
Randomization occurred immediately after consent was obtained
preoperatively and was performed using permuted blocks of random
length by the independent Clinical Research Database of
fi
ce, which
ensured concealment of allocation with a password-protected database.
Assignments were communicated via telephone or encrypted email to
the pharmacy. With the exception of the hospital pharmacists who
dispensed the study drugs, all participants were masked to the treatment
assignments.
The protocol was approved by the Memorial Sloan Kettering
Institutional Review Board. All patients provided written informed
consent before enrollment and surgery. The formal trial protocol can be
found in the Supplementary data.
[(Fig._1)TD$FIG]
Assessed for eligibility (
n
= 459)
Excluded (
n
= 249)
•
Did not meet inclusion criteria (
n
= 62)
•
Declined to participate (
n
= 122)
Randomized (
n
= 210)
Allocated to mannitol (
n
= 105)
•
Received mannitol (
n
= 101)
•
Surgery (
n
= 4)
Allocated to placebo (
n
= 105)
•
Received placebo (
n
= 98)
•
Surgery (
n
= 5)
•
Surgery cancelled (
n
= 2)
Analyzed (
n
= 101)
Analyzed (
n
= 98)
Not approached (
n
= 65)
•
MD discretion (
n
= 33)
•
Clinic delay (
n
= 2)
•
Bilateral RCC (
n
= 3)
•
No ischemia anticipated (
n
= 4)
•
No preop renal scan (
n
= 11)
•
Possible radical Nx (
n
= 3)
•
Solitary kidney (
n
= 5)
•
Did not want to discuss research
(
n
= 4)
Fig. 1
–
Flow diagram for enrollment and randomization of trial.
MD = medical doctor; preop = preoperative; RCC = renal cell carcinoma.
E U R O P E A N U R O L O GY 7 3 ( 2 0 18 ) 5 3
–
5 9
54
















