
angiogenesis (hypoxia inducible factor 1
a
and metallopro-
teinases), cell proliferation (Ki-67), epithelial-mesenchymal
transition (Snail), mitosis (Aurora A), apoptosis (Bcl-2 and
survivin), vascular invasion (RON), and c-met protein (MET)
[1,29,47] .Microsatellite instability (MSI) is an independent
molecular prognostic marker
[48]. MSI can help detect
germline mutations and hereditary cancers
[10]. Because of
the rarity of UTUC, the main limitations of the above studies
are their retrospective design and small sample size. None of
the markers have fulfilled the criteria necessary to support
their introduction in daily clinical decision making.
3.4.3.
Predictive tools
Accurate predictive tools are rare for UTUC. There are two
models in the preoperative setting: one for predicting LND
of locally advanced cancer that could guide the decision to
perform, or not, an LND as well as the extent of LND at the
time of RNU
[1] ,and one for the selection of non
–
organ-
confined UTUC that is likely to benefit from RNU
[49]. Four
nomograms are available predicting survival rates postop-
eratively, based on standard pathological features
[1,50,51].
3.4.4.
Bladder recurrence
A recent meta-analysis of available data has identified
significant predictors of bladder recurrence after RNU
[52](LE: 3). Three categories of predictors of increased risk for
bladder recurrence were identified:
1. Patient-specific factors such as male gender, previous
BCa, smoking and preoperative chronic kidney disease
2. Tumour-specific factors such as positive preoperative
urinary cytology, ureteral location, multifocality, invasive
pT stage, and necrosis
3. Treatment-specific factors such as laparoscopic ap-
proach, extravesical bladder cuff removal, and positive
surgical margins
[52]In addition, the use of diagnostic ureteroscopy has been
associated with a higher risk of developing bladder
recurrence after RNU
[53](LE: 3).
3.4.5.
Risk stratification
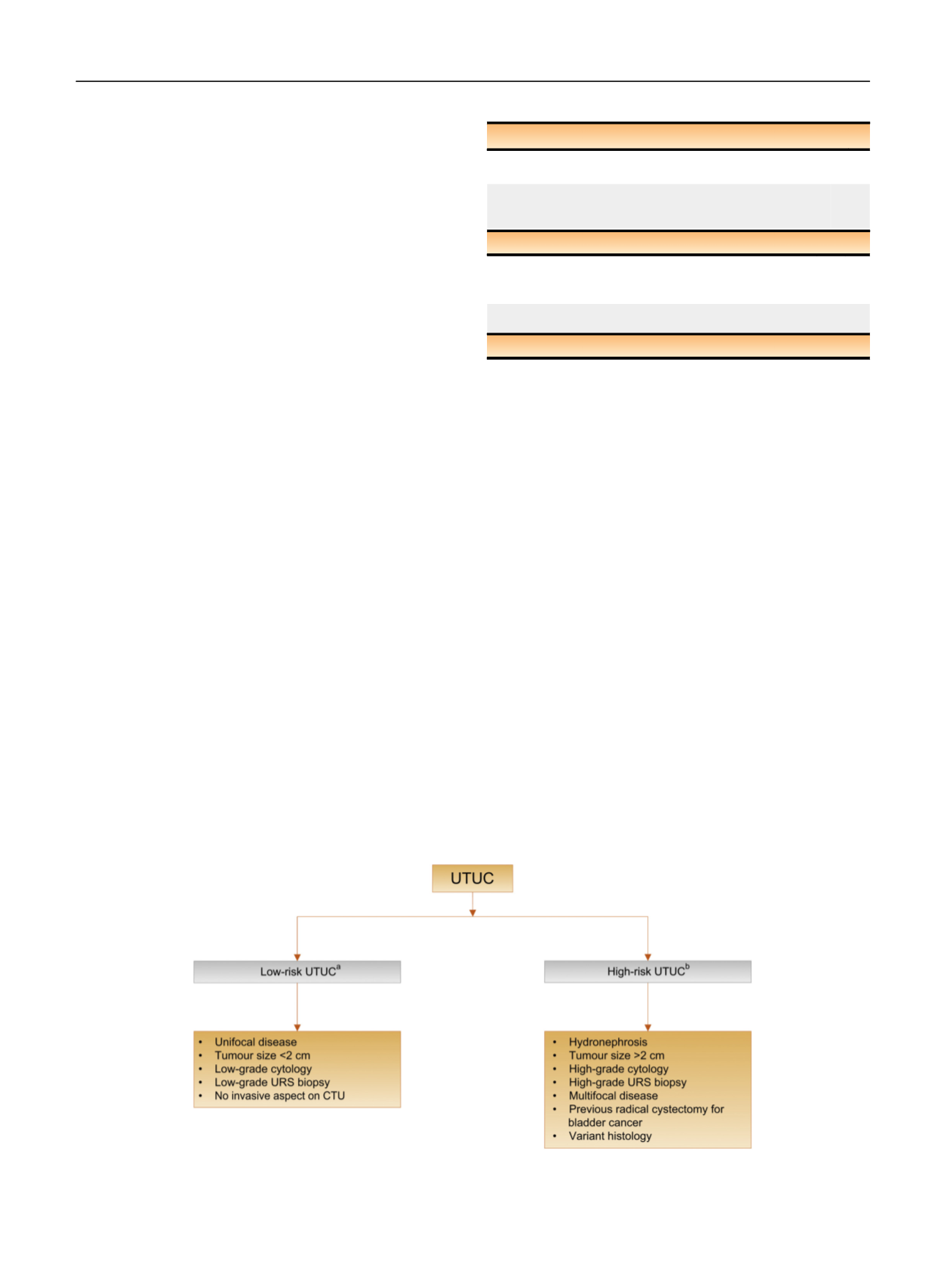
As tumour stage is difficult to assert clinically in UTUC, it is
useful to
“
risk stratify
”
UTUC between low- and high-risk
tumours to identify those who are more suitable for kidney-
sparing treatment rather than radical extirpative surgery
[1] ( Fig. 3).
3.5.
Disease management
3.5.1.
Localised disease
3.5.1.1. Kidney-sparing surgery.
Kidney-sparing surgery (KSS) for
low-risk UTUC allows sparing the morbidity associated with
radical surgery, without compromising oncological out-
comes and kidney function
( Table 3 ) [1] .In low-risk cancers,
it is the preferred approach with survival being similar after
KSS versus RNU
[54] .This option should therefore be
discussed in all low-risk cases, irrespective of the status of
the contralateral kidney. In addition, it can also be
considered in select patients with serious renal insufficiency
or solitary kidney (LE: 3). Recommendations for kidney-
sparing management of UTUC are listed in
Table 4.
3.5.1.1.1. Ureteroscopy.
Endoscopic ablation can be considered
in patients with clinically low-risk cancer in the following
situations
[1,55]:
Table 3
–
Summary of evidence and guidelines for prognosis
Summary of evidence
LE
Age, sex, and ethnicity are no longer considered as independent
prognostic factors.
3
Primary recognised postoperative prognostic factors are tumour
stage and grade, extranodal extension, and lymphovascular
invasion.
3
Recommendations
LE GR
Use microsatellite instability as an independent molecular
prognostic marker to help detect germline mutations
and hereditary cancers.
3 C
Use the American Society of Anesthesiologists score to assess
cancer-speci
fi
c survival following surgery.
3 C
GR = grade of recommendations; LE = level of evidence.
[(Fig._3)TD$FIG]
Fig. 3
–
Risk stratification of upper urinary tract urothelial carcinoma. CTU = computed tomography urography; URS = ureteroscopy; UTUC = upper
urinary tract urothelial carcinoma.
a
All these factors need to be present.
b
Any of these factors need to be present.
E U R O P E A N U R O L O GY 7 3 ( 2 0 18 ) 111
–
1 2 2
116
















