
risk of tumour recurrence, (3) means of diagnosis of tumour
recurrence, (4) duration of free period prior to transplanta-
tion, and (5) tumour-free status prior to transplantation. For
each study, it was assessed whether each confounder was
considered and whether, if necessary, the confounder was
controlled in analysis. The RoB was considered to be high if
the confounder had not been considered, was imbalanced
between patients or not corrected for during analysis. This
approach is detailed in the study protocol (PROSPERO).
2.6.
Data synthesis
Methodological and clinical heterogeneity of the included
studies meant that meta-analysis was inappropriate,
therefore, a narrative synthesis of the data was performed
( https://www.york.ac.uk/crd/guidance/). The primary out-
comewas cancer recurrence at
<
1-yr,1
–
5-yr, and
>
5-yr time
points and is summarised in descriptive tables. Secondary
outcomes were cancer-specific and the overall survival at
<
1-yr, 1
–
5-yr, and
>
5-yr time points. Possible reasons for
heterogeneity were explored using the available information
such as differences in the population studied, the treatment
given, or the way in which the outcomes were assessed.
Intended formal subgroup analysis was not possible due to
the inclusion of nonrandomised comparative studies.
Therefore, any subgroup differences were discussed narra-
tively to explore potential effect size differences. A planned
sensitivity analysis to assess the robustness of our review
results by repeating the analysis only including studies with
an overall medium to low RoB, was not possible.
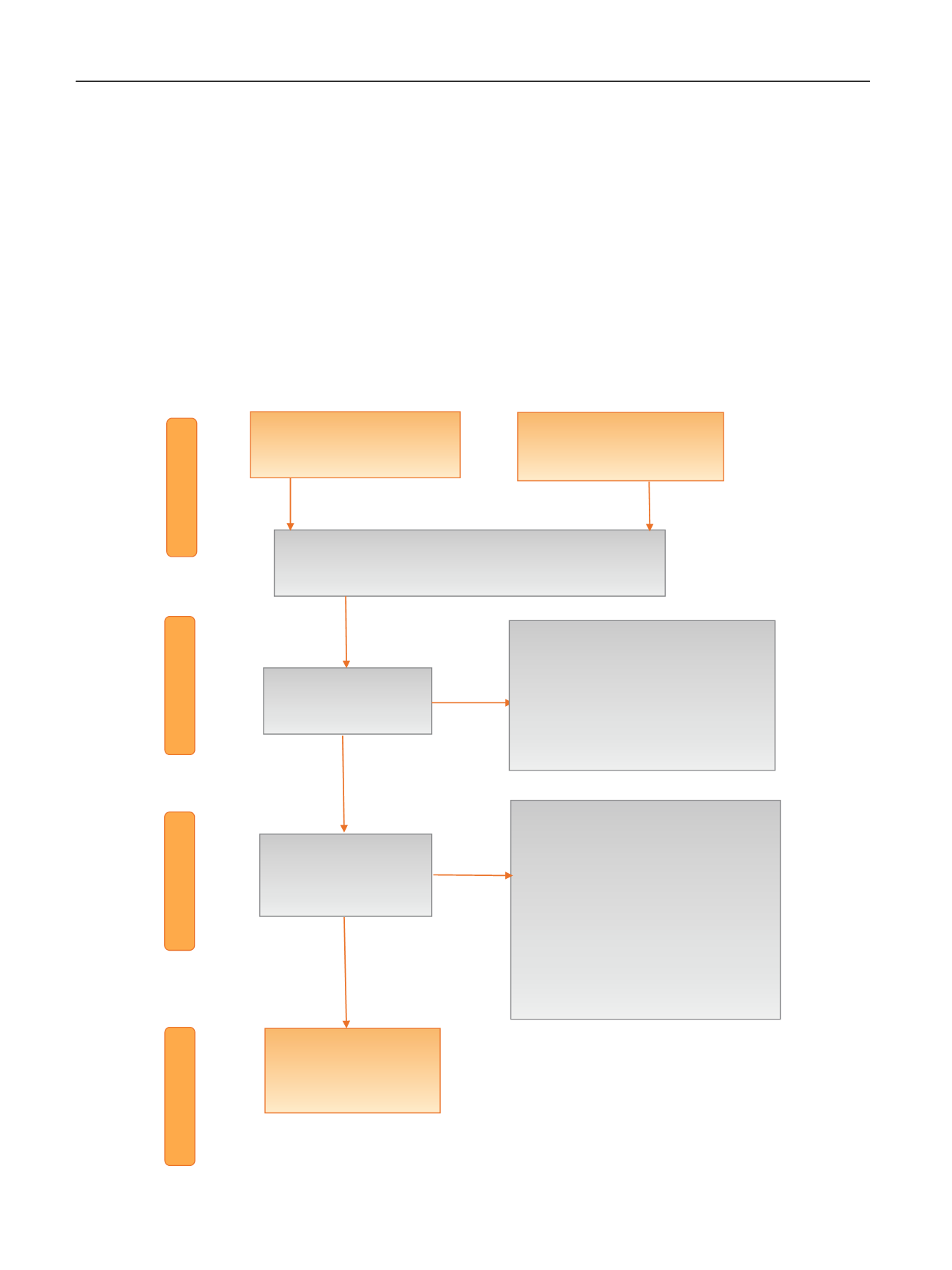
[(Fig._1)TD$FIG]
Screening
Included
Eligibility
Records screened
(
n
= 1891)
Full-text articles assessed for
eligibility
(
n
= 72)
Studies included in
qualitative synthesis
(
n
= 32)
Records identified through
database searching (
n
= 3308)
Records excluded (
n
= 40)
•
Reviews (
n
= 5)
•
Period of inclusion before 1995 (
n
= 2)
•
Outcome of interest not reported (
n
=
13)
•
Cancer diagnosed after renal
transplantation (
n
= 9)
•
Nonurological cancer (
n
= 3)
•
Duplicate study (
n
= 1)
•
Older publications on same series (
n
=
7)
Identification
Records excluded (
n
= 1819)
•
Abstract only or publication before
1995 (
n
= 119)
•
Invalid population (
n
= 1689)
•
No renal transplantation or renal
replacement therapy (
n
= 5)
•
No oncological data (
n
= 6)
Records after duplicates removed
(
n
= 1891)
Additional search in references of
included studies (
n
= 19)
Fig. 1
–
Preferred reporting items for systematic reviews and meta-analyses flow chart.
E U R O P E A N U R O L O GY 7 3 ( 2 0 18 ) 9 4
–
10 8
96
















